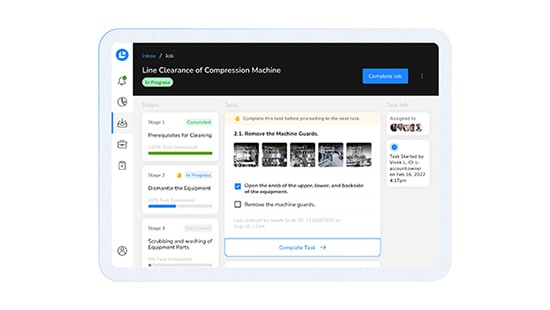
CLEEN Digital Logbook Module
A purpose-built digital logbook for pharmaceutical and personal care operations that goes beyond the standard logbook to rapidly convert any paper form process into a self-enforcing digital workflow with a comprehensive audit trail for confident data integrity.
Digitize Essential Workflows
To Drive Performance and Compliance
Add a Descriptive and SEO Subtitle Here

Move Away From Paper
Digitize the processes throughout your facility to enable real-time performance tracking, customized interlocks, and create self-enforcing workflows to ensure compliance. Include embedded videos or images to guide operators through instructions.
Proactively Manage Compliance
Receive real-time visibility into each step of a process, ensuring precise tracking of actions taken - including alerts designed to notify users of newly flagged reviews and any emerging issues that may require attention.
Optimize Operations
Use performance benchmarking and embedded analytics to uncover process steps taking the most time as well as opportunities to improve operations—reducing downtime, improving equipment availability, and increasing the efficient utilization of resources.
Key Benefits
Efficient, Paper-Free Processes
- Reduce Manual Processes: convert paper forms, SOPs and logbooks into digital logbooks with a no-code process builder.
- Automatic Timestamp Capture: Record timestamps automatically to ensure accurate data logging.
- System Integration: Integrate with ERP systems, like SAP for real-time product and material data access.
- QR Code Utilization: Quickly update records through QR code scanning for enhanced efficiency.

Error Reduction and Compliance Assurance
- Self-Enforcing Interlocks: Enforce critical process controls to adhere to ALCOA principles helping ensure data is attributable, legible and accurate.
- Automated Compliance Checks: Incorporate compliance verification into digital workflows to ensure SOP adherence without manual intervention.

Audit Preparedness
- Automated Report Generation: Generate required documentation and reports on demand to support inspection readiness.
- Activity Tracking: Maintain a comprehensive log of all actions and changes within the platform for accountability.
- Centralized Documentation Access: Access protocols, reports, SOPs, and supporting documents from a single digital repository for efficient retrieval during audits.
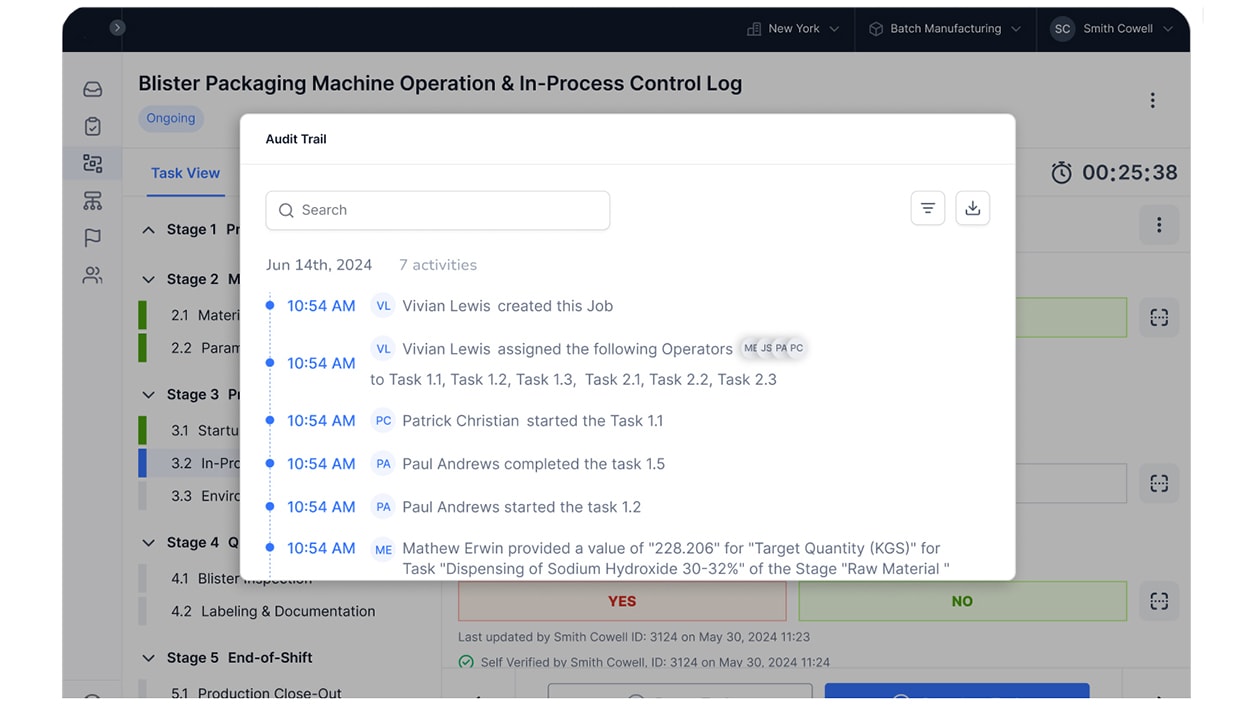
Improve Data, Reduce Risk, Monitor Parameters with CLEEN
Out-of-the-Box Data Connections for Rapid Integrations
ERP | QMS | LMS | DMS | SSO | EM
Security Certifications and Compliance
Compliant and Scalable Cloud Infrastructure
GxP Compliant, ISO 27001 Certified
Data Centers
- Data centers are certified by 3rd party auditors for ISO 9001:2015, ISO 27001
- GxP guidelines available and implemented
SSO and LDAP
- Single sign-on (SSO) and lightweight directory access protocol (LDAP) allow you to authenticate users in systems
- Configurable by customer IT team
Vulnerability Assessment and
Penetration Testing
- Periodic VAPT
- Performed by certified 3rd party
- Results shared with customers on-demand
End-to-End Data Encryption
for Virtual Machines
- Server side encryption with PMK
- Data sent to or from Leucine is encrypted in transit using 256 bit encryption
Domain Whitelisting
- Allow or disallow domains that can access the platform
- Configure by customer IT team

Backup and Disaster Recovery
- Daily backups available
- Disaster recovery Regions
- Complete and incremental backups

Embracing Pharma 4.0
Why operational digitization must be the top priority for pharma and biopharma manufacturers.
This free eBook outlines the fundamental shifts associated with embracing Pharma 4.0—and how they can empower organizations to improve efficiency, agility, and maintain product quality while driving business profitability.


