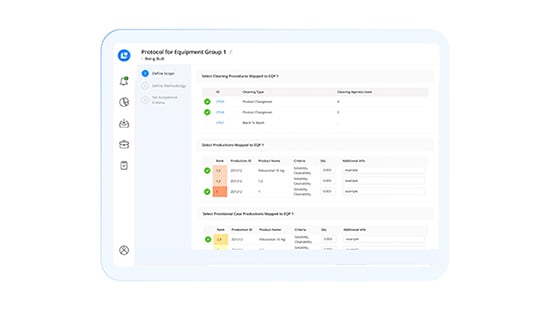
CLEEN Validation Module
Streamline, automate and standardize cleaning validation for life sciences organizations.
Modernize Cleaning Validation With Intelligent Automation
Add a Descriptive and SEO Subtitle Here
Ensure Compliant Validations
Receive in-platform guidance as you build cleaning validations based on continuously updated regulations. Automatically calculate carryover limits and worst-case product rankings, and leverage automated compliance interlocks for CHT/DHT.
Accelerate Re-Validation
Leverage historical data to calculate carryover limits and create protocols in minutes by integrating data from multiple sources, speeding up new product introductions. Receive dynamic alerts for necessary re-validations complete with auto-generated documents for review.
Simplify Audit-Readiness
Automatically generate validation reports and store all necessary information in the built-in Audit Portal for immediate access that enables continuous audit-readiness with minimal administrative burden.
Key Features
Generate and Execute Protocols
- Instant Protocol Generation: Quickly run validation calculations and auto-generate cleaning validation protocols in seconds, minimizing time spent on protocol development from weeks to minutes.
- Multi-Source Data Integration: Consolidate information from multiple data sources into new protocols with a single click, streamlining the validation process.
- Impact Assessment: Evaluate how the introduction of new products or equipment affect your cleaning validation protocols, ensuring ongoing compliance with the latest regulatory standards.

Comprehensive Digital Audit Trail
- Immediate Access to Materials: Maintain a complete digital audit trail with instant access to all necessary supporting documents, ensuring confidence in data integrity.
- Inspector Walkthrough: Facilitate smooth audit experiences by easily guiding auditors through your cleaning validation protocols and adherence to guidelines.

Risk-Based Recommendations
- Identify Trends and Issues: Utilize historical data to anticipate potential problems, enhancing your risk-based cleaning validation approach.
- Worst-Case Scenario Analysis: Determine carryover limits and evaluate the hardest-to-clean ingredients within your facility, helping ensure comprehensive cleaning validation.

Out-of-the-Box Data Connections for Rapid Integrations
ERP | QMS | LMS | DMS | SSO | EM
Security Certifications and Compliance
Compliant and Scalable Cloud Infrastructure
GxP Compliant, ISO 27001 Certified
Data Centers
- Data centers are certified by 3rd party auditors for ISO 9001:2015, ISO 27001
- GxP guidelines available and implemented
SSO and LDAP
- Single sign-on (SSO) and lightweight directory access protocol (LDAP) allow you to authenticate users in systems
- Configurable by customer IT team
Vulnerability Assessment and
Penetration Testing
- Periodic VAPT
- Performed by certified 3rd party
- Results shared with customers on-demand
End-to-End Data Encryption
for Virtual Machines
- Server side encryption with PMK
- Data sent to or from Leucine is encrypted in transit using 256 bit encryption
Domain Whitelisting
- Allow or disallow domains that can access the platform
- Configure by customer IT team

Backup and Disaster Recovery
- Daily backups available
- Disaster recovery Regions
- Complete and incremental backups

Embracing Pharma 4.0
Why operational digitization must be the top priority for pharma and biopharma manufacturers.
This free eBook outlines the fundamental shifts associated with embracing Pharma 4.0—and how they can empower organizations to improve efficiency, agility, and maintain product quality while driving business profitability.

Avoiding Top FDA Form 483 Observations in Pharma Manufacturing
Why proactive measures must be the top priority for pharma manufacturers.
This comprehensive article outlines the critical observations that can be avoided in pharma manufacturing and how taking proactive measures can enhance compliance, ensure product quality, and maintain regulatory standards while optimizing operational efficiency.

